Cannabinoids are chemical compounds that interact with the human body's endocannabinoid system. This system plays a key role in regulating functions such as pain, sleep, mood, and appetite. Cannabinoids are primarily found in cannabis but have also been discovered in other parts of nature, such as other plants and fungi.
The endocannabinoid system consists of receptors (CB1 and CB2), endocannabinoids (molecules produced by the body), and enzymes that break down these compounds. When cannabinoids bind to these receptors, they can influence a wide range of physiological functions, providing therapeutic effects on both the body and mind.
Types of cannabinoids
The Cannabis sativa plant contains over 400 chemical substances, including numerous cannabinoids. Cannabis plants produce more than 100 different cannabinoids. Similarly, various spices and aromatic herbs contain compounds that can interact with the endocannabinoid system.
Cannabinoids can be classified into three main types: phytocannabinoids (derived from cannabis or plant extracts, such as THC, CBD, CBG, CBN, or CBDV), endocannabinoids, and synthetic cannabinoids.
The study A Novel Phytocannabinoid Isolated from Cannabis sativa L., published in the journal Scientific Reports, identified two new cannabinoids: tetrahydrocannabiphorol (THCP) and cannabidiphorol (CBDP). THCP, in particular, has captured the attention of the scientific community due to its chemical structure, which gives it up to 30 times greater binding affinity to CB1 receptors compared to THC.
This high affinity suggests that THCP could have significantly stronger psychoactive effects than THC, although further studies are needed to fully understand its implications and potential therapeutic applications.
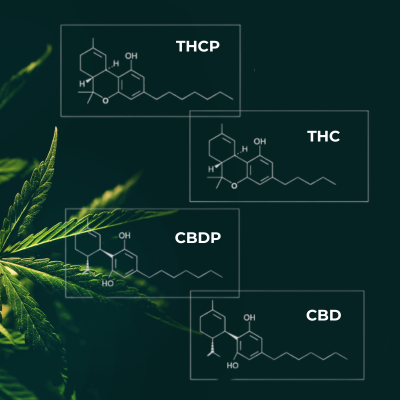
On the other hand, CBDP has shown a slightly weaker binding affinity to CB1 and CB2 receptors compared to CBD. However, recent research indicates that, like CBD, CBDP can act on the 5-HT1A receptor, potentially contributing to anxiolytic effects. Its interaction with other receptors, such as dopamine receptors, differs from CBD. This finding opens new possibilities for exploring how CBDP can be used in therapies for conditions such as anxiety and chronic pain, although more research is required to fully understand its effects (International Journal of Molecular Sciences, 2024).
Recent studies have also discovered that cannabinoids are not only found in plants but also in other biological kingdoms, such as fungi. These compounds, known as mycocannabinoids, are being studied for their potential to influence the endocannabinoid system.
Phytocannabinoids
Phytocannabinoids are cannabinoids naturally found in plants, with cannabis being the most well-known source. This group includes compounds such as tetrahydrocannabinol (THC) and cannabidiol (CBD), which are the most studied and used for their psychoactive and therapeutic effects.
Phytocannabinoids are produced in the glandular trichomes of cannabis plants, which are microscopic structures found on the surface of its leaves, flowers, and stems. These glands secrete resins containing phytocannabinoids, terpenes, and other bioactive compounds. The plant generates phytocannabinoids as part of its defense mechanism, protecting it from pests, pathogens, and adverse environmental conditions.
There are different types of trichomes in cannabis. Non-glandular trichomes, such as simple unicellular trichomes and cystolithic trichomes, are not associated with the production of terpenoids. However, glandular trichomes are the main players in phytocannabinoid synthesis. In female plants, three main types have been identified: bulbous trichomes, sessile capitate trichomes, and stalked capitate trichomes, the latter being the most abundant in phytocannabinoids. Male plants feature a unique fourth type, glandular anther trichomes, found exclusively in the anthers.
Although trichomes are present throughout the plant, the highest concentrations of phytocannabinoids are found in the bracts of female flowers, reaching up to 20-25% of dry weight. These stalked capitate trichomes primarily develop during flowering and are most densely concentrated in the bracts of pistillate flowers, extending to the small leaves surrounding them. In contrast, phytocannabinoid levels are significantly lower in large leaves and stems, and virtually absent in roots.
While there are no qualitative differences in the phytocannabinoid profile between various parts of the plant, there are quantitative variations. Regarding their function in the plant, phytocannabinoids are believed to play a defensive role, protecting it against biotic stress (such as insects, bacteria, and fungi) and abiotic stress (such as dehydration and ultraviolet radiation).
Endocannabinoids
The endocannabinoid system (ECS) is a complex network of receptors, endocannabinoids, and enzymes that plays a crucial role in regulating various physiological and cognitive functions in the human body. The two main endocannabinoids are anandamide (AEA) and 2-arachidonoylglycerol (2-AG).
These endogenous compounds are produced as needed by the body and bind to CB1 and CB2 receptors, modulating processes such as pain, appetite, mood, and memory. These endocannabinoids act on the same receptors as phytocannabinoids but are generated by the body to maintain homeostatic balance in various biological functions.
Recent research has revealed that ECS dysfunction is associated with various pathologies, including metabolic disorders such as obesity and diabetes mellitus. For example, a study published in Revista Biomédica highlights that overstimulation of the ECS can disrupt lipid metabolism, contributing to the development of these conditions.
Additionally, the ECS influences the endocrine system, affecting hormone release and processes such as growth, metabolism, and reproduction. An article in Revista SAEGRE discusses how endocannabinoids interact with endocrine glands, modulating their function and affecting bodily homeostasis.
Synthetic Cannabinoids
Synthetic cannabinoids are compounds artificially created in laboratories to mimic the effects of natural cannabinoids. These products are specifically designed to interact with the receptors of the endocannabinoid system, similar to cannabis-derived phytocannabinoids.
Unlike natural cannabinoids, synthetics can be much more potent and cause severe side effects, as they are often not subject to strict regulations and may affect the body unpredictably.
The consumption of synthetic cannabinoids can cause severe side effects, including tachycardia, hypertension, seizures, psychotic episodes, and, in extreme cases, death. There have also been reports of mass poisoning outbreaks. Notable examples include the MDMB-FUBINACA incident in Russia, which caused numerous hospitalizations and deaths, and the "zombie outbreak" caused by AMB-FUBINACA in New York on July 12, 2016.
This event involved 33 people intoxicated by AMB-FUBINACA, a potent cannabinoid receptor agonist, whose victims exhibited severe symptoms, leading to comparisons to a "zombie outbreak" due to their altered appearance. Such incidents highlight the inherent dangers of consuming synthetic cannabinoids, which can have unpredictable and dangerous health effects.
Mycocannabinoids
Mycannabinoids, discovered in certain fungal species, are molecules similar to phytocannabinoids but originate from a different biological kingdom: fungi. Fungi containing mycannabinoids include species such as reishi, cordyceps, and turkey tail, which are known for their medicinal properties.
Although research in this field is still in its early stages, recent studies have shown that certain fungi contain compounds capable of interacting with CB1 and CB2 receptors.
These compounds may have effects similar to phytocannabinoids, such as reducing inflammation, relieving pain, and enhancing overall well-being.

How do cannabinoids interact with the endocannabinoid system?
The endocannabinoid system is fundamental for regulating many biological processes in the human body. Cannabinoids, whether endogenous (produced by the body) or exogenous (from plants or fungi), interact with CB1 and CB2 receptors in the brain, central nervous system, peripheral organs, immune system, and other tissues.
When a cannabinoid binds to one of these receptors, it triggers a biological response that can have therapeutic effects on various body functions. For example, THC activates CB1 receptors in the brain, causing psychoactive effects, while CBD interacts more with CB2 receptors, explaining its anti-inflammatory and relaxing properties.
The role of cannabinoids in our cells
Mitochondria are fundamental organelles in our cells responsible for producing energy in the form of ATP (adenosine triphosphate) and regulating functions such as calcium homeostasis, apoptosis, and metabolism.
Mitochondria have their own DNA, inherited exclusively from the mother, and any dysfunction can trigger diseases affecting organs with high energy demands, such as the heart, brain, and muscles.
Recent research has discovered that the outer mitochondrial membrane contains CB1 receptors, confirming that cannabinoids can directly influence mitochondrial function and cellular energy production. Moreover, cannabinoids act as signaling molecules, affecting energy metabolism and communication between the cell nucleus and mitochondria.
For instance, cannabinoids can reduce ATP production, which may be beneficial in inhibiting tumor cell proliferation but could also suppress immune functions in other contexts.
Mitochondrial protection and CBD
CBD, in particular, has been shown to be a potent mitochondrial protector, acting as an antioxidant and neutralizing damage caused by free radicals. This explains why CBD is effective in treating seizures, as it regulates dysfunctional calcium signaling originating in mitochondria.
In nerve cells, CBD can balance activity, reducing excessive excitation caused by stress or toxins, and protecting neurons by stabilizing mitochondrial function.
The study Neuroprotective Effects of Cannabinoids in Neurodegenerative Diseases has confirmed that CBD, along with other cannabinoids, can protect nerve cells, specifically safeguarding striatal neurons from toxicity induced by mitochondrial inhibitors such as 3-nitropropionic acid (3NP), which generates oxidative stress. These studies suggest that cannabinoids not only have anti-inflammatory properties but also neuroprotective and neuroregenerative effects, expanding their therapeutic potential for neurodegenerative diseases.
Cannabinoids and glaucoma
Cannabinoids have sparked interest in treating glaucoma, a disease that affects more than 60 million people and can cause permanent blindness.
Δ9-tetrahydrocannabinol (THC), the main component of cannabis, reduces intraocular pressure by activating receptors such as CB1 and GPR18. This effect varies depending on sex and the time of day, while cannabidiol (CBD) increases intraocular pressure and blocks THC’s effects, complicating its use in glaucoma patients.
Despite these challenges, endogenous cannabinoids offer a promising avenue for developing new treatments. Preventing their breakdown could effectively regulate intraocular pressure.
A recent study, Cannabis Use and the Risk of Primary Open-Angle Glaucoma: A Mendelian Randomization Study, published in Nature, investigates how cannabinoids can be effective not only in regulating intraocular pressure (IOP) but also in protecting retinal ganglion cells (RGCs), which are crucial for vision.
Researchers found that cannabinoids such as THC and CBD may have neuroprotective properties that help reduce neuronal damage in glaucoma patients. This discovery goes beyond the traditional effects of cannabinoids in reducing IOP. The study demonstrated that these compounds not only influence intraocular pressure but also protect retinal nerve cells against oxidative stress and neuronal inflammation, which are key factors in the damage that leads to vision loss in glaucoma.